The National Blood Authority has released its latest haemovigilance report. Check out its findings.
The number of transfusion-related adverse events decreased in 2021-22, but the number of events that occurred as a result of not following proper hospital procedure doubled.
Between July 2021 and June 2022 Australian hospitals reported 659 transfusion-related adverse events, a decrease on the 706 reported in 2020-21 and the 675 reported in 2019-20.
Of the 659 adverse events, 75 were classified as serious adverse events, defined as an event that is possibly, probably, or confirmed to be related to a blood transfusion and results in severe morbidity or death.
Adverse events occurred at similar rates in males and females (49% versus 50%; 1% of events did not have report the sex of the patient) but were more common in older patients compared to younger patients – 2% of all adverse reactions occurred in patients aged 0-4 years, which increased to 26% in patients aged 75 and older.
Although 526 hospitals (424 of which are public) participate in national haemovigilance reporting, only a quarter of these (96 public and 33 private) indicated they experienced an adverse event during the reporting period.
The number of adverse events varied between states and territories.
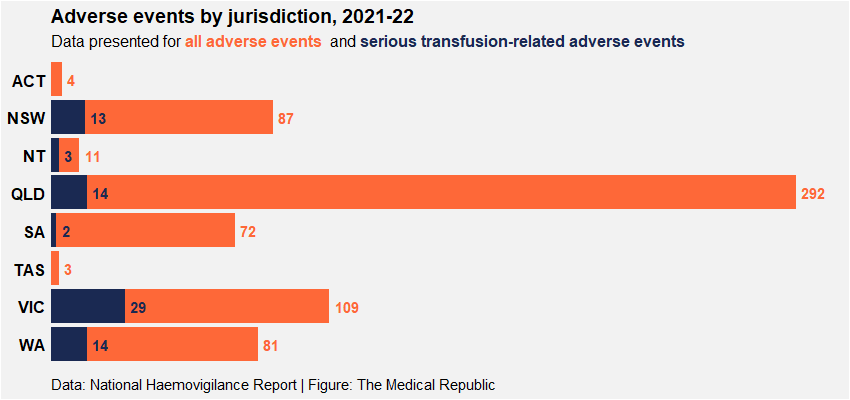
A greater proportion of adverse events occurred in public compared to private hospitals (87% versus 13%), although this was not surprising given the larger number of public hospitals contributing data to the report.
“Ony three states (Victoria, Queensland and Western Australia) reported adverse events [in] private hospitals, [while] Queensland had the highest number of reporting and participating hospitals for private hospitals,” the report authors noted.
“[However,] private hospitals from New South Wales and South Australia did not participate in national haemovigilance reporting…The use of different haemovigilance reporting processes across the jurisdictions may lead to data inconsistencies.”
Febrile non-haemolytic transfusion reactions (38%) and allergic/anaphylactic reactions (27%) were the most commonly reported adverse events, ahead of transfusion-associated circulatory overload (13%) and the incorrect blood component being transfused (6%).
Transfusion-associated circulatory overload and allergic/anaphylactic reactions were the most common transfusion-related serious adverse events (36% each).
When the factor(s) contributing to each adverse event were considered, “product characteristic” (187 mentions) and “none identified” (179) were the most commonly identified factors.
Related
The number of adverse events with no identifiable contributing factor almost halved compared to the previous reporting period. In contrast, “administration of product” (121, up from 60 in the previous reporting period), “deliberate clinical decision” (110, up from 57) and “procedure did not adhere to hospital transfusion guidelines” (44, up from 22) were all significantly more common during the 2021-22 reporting period.
Over 60% of adverse events resulted in minor morbidity and 8% in severe morbidity, while 4% of events were deemed to be life-threatening. There were three deaths as a result of adverse events. The majority (71%) of adverse events were related to red cell transfusions.
The complete report can be freely accessed from the National Blood Authority’s website. The 2022-23 edition of the report is expected to be released later this year.